Basic knowledge of corrosion
There are countless metal products around us, such as car bodies, door hinges, screws and bolts. Corrosion such as rust may occur in metal products depending on the usage environment and aging, which may lead directly to serious accidents. According to survey results, corrosion-related costs reach approximately 3% (approximately 15 trillion yen) of GDP, making corrosion countermeasures an important issue for the manufacturing and construction industries. In this six-part series, we will explain the basic knowledge of corrosion that engineers should know.
Contents
Part 1: What is Corrosion?
Corrosion is a phenomenon in which metal materials are degraded or damaged by chemical processes. Let's take iron as an example and think about corrosion. Iron occurs in nature as iron ore (iron oxide). Iron ore is the most stable state on earth where water and oxygen are plentiful. Iron as a material (steel manufacturing) is produced by forcibly separating oxygen from iron ore using a reducing agent such as coke in a blast furnace. This process is called smelting. The metal element iron that is extracted is extremely unstable in the natural environment, so it always tries to return to iron oxide. This reaction is the corrosion reaction and rust is produced. Chemically, rust is the same as iron ore. Corrosion and refining are in the same reaction system, even though they show opposite directions (Fig. 1).
Figure 1: Oxidation and reduction reactions of ironWhen iron is put into a strong acid such as hydrochloric acid, it dissolves into an aqueous solution as iron ions while generating hydrogen gas. This reaction occurs as a combination of the ionization reaction of iron (oxidation reaction) and the reduction reaction of hydrogen ions in the environment (hydrogen gas generation reaction), and is represented by the following electrochemical formula.
1: Fe→Fe2++2e–
2: 2H++2e–→H2 (hydrogen gas generation)
When formula 1 and formula 2 are added, it becomes a notation by a normal chemical formula.
3: Fe+2H+ → Fe2++ H2
The rusting reaction of iron in the natural environment is also expressed in the same electrochemical formula, except that the reaction corresponding to Equation 2 is the reduction reaction of oxygen.
4: O2+2H2O+4e–→4OH–
If you multiply equation 1 by 2 and add it with equation 4, you get the iron oxidation reaction equation.
5: 2Fe+O2+2H2O→2Fe(OH)2
When some water molecules are extracted from the generated iron hydroxide Fe(OH)2, or oxidation by oxygen in the air occurs, iron oxide Fe 2O3 or Fe3O4. This is what rust is. The phenomenon in which iron ionizes and dissolves in an acidic solution and the phenomenon in which iron corrodes and rusts in a neutral environment are essentially the same corrosion reactions. Both can be represented by electrochemical reaction equations.
The local cell model of corrosion is a model such as Fig. 2 that describes corrosion. Figure 2 shows the corrosion of iron in an acidic solution. The cathode is where hydrogen ions are reduced and hydrogen gas is generated, and the anode is where iron is ionized and dissolved. Electrons generated at the anode move to the cathode and are consumed by the hydrogen gas generation reaction. Electron flow and current flow in opposite directions.
Figure 2: Corrosion Local Cell ModelIn reality, the locations where these electrochemical reactions occur (anode, cathode) are not fixed specific locations, but fluctuate from time to time, …
>>Read the continuation of Part 1 Chapter 2 (PDF download)
Redox potential (potential) and pH are fundamental environmental factors that affect metal corrosion in aqueous solutions. This is because the corrosion reaction is a redox reaction and the solubility (solubility and dissolution rate) of metals and their oxides is greatly affected by pH. A diagram conceptually showing which chemical species are stable, with potential on the vertical axis and pH on the horizontal axis, is called a potential-pH diagram or Pourbaix diagram (Fig. 3). Chemical species is a general term for substances that affect chemical reactions, such as ions, atoms, and elements.
Figure 3: Potential-pH diagram (Pourbaix diagram)The reaction tendency of metals in each region of the potential-pH diagram differs from region to region.
・Inactive region: Extremely reducing environment. Single metal is stable and does not corrode.
・Corrosion (active state) region: Highly oxidizing region. Metal ions are stable on the low pH side, so corrosion occurs.
・Passive region:……
>>Read the continuation of Part 1 Chapter 3 (PDF download)
Passivation is a corrosion phenomenon in which the corrosion rate of metal placed in an oxidizing environment is extremely slow (corrosion resistance is improved). Oxidation (corrosion) progresses when metal is placed in an oxidizing environment. At this time, the dissolved metal ions deposit as a dense thin oxide film, which covers the metal surface. The oxide film is only a few nanometers thick and is in a dynamic state, constantly repeating dissolution and regeneration. Passive corrosion resistance is...
>>Read the continuation of Chapter 4 (PDF download)
Corrosion can take many forms. By learning why each type of corrosion occurs, you can take appropriate countermeasures. The characteristics and causes of occurrence of 10 types of corrosion forms are summarized.
General corrosion is a form of corrosion seen when iron is corroded by strong acid, and the corroded surface is relatively uniform. It is also seen when stainless steel cannot be passivated and corrodes in the active state.
Dissimilar metal galvanic corrosion is a form of corrosion that occurs when dissimilar metals are brought into contact with each other and exposed to a corrosive environment. Attracted by noble metals (metals with low ionization tendency), corrosion of base metals (metals with high ionization tendency) is accelerated. The ionization tendency is as follows.
K>Ca>Na>Mg>Al> Zn>Fe>Ni> Sn>Pb>(H)>Cu>Hg>Ag>Pt>Au Therefore, it is included as a reference.
The ionization tendency is large (base) on the left side, and the ionization tendency is small (noble) toward the right side. Let's remember, "Let's lend it, but it's too much debt to rely on." Even if the metal is the same, the outside of the passive state is noble, and the inside of the local anode is noble, and localized corrosion may occur by the galvanic corrosion mechanism.
Pitting corrosion is corrosion that can be seen in materials such as stainless steel in environments containing chloride ions, such as seawater and saline solutions. The coating in specific areas is destroyed, and corrosion progresses in pits. It occurs only when it has a strong oxidizing property to some extent, and pitting corrosion does not occur in a reducing environment. In the pits, the dissolved metal ions are concentrated, and hydrolysis produces hydrogen ions (acids), further increasing corrosiveness, creating a vicious cycle.
Crevice corrosion is a form of corrosion that occurs when the passive film breaks when the same metals are placed on top of each other or when foreign matter adheres to the surface. It occurs in stainless steel, etc., with the same mechanism as pitting corrosion. Since it does not require an oxidizing environment, it is more common than pitting and is an important corrosion problem.
Stress corrosion cracking is a form of localized corrosion that occurs when metal under tensile stress is exposed to a chloride environment. It occurs in stainless steel, etc., and the main source of stress is residual stress that occurs during welding and machining. It can be prevented by removing residual stress by stress relief annealing.
……
>>Read the continuation of Part 1 Chapter 5 (PDF download)
Part 2: Corrosion of Aluminum, Stainless Steel, and Copper
In the previous article, I explained the mechanisms and types of corrosion. This time, I will explain the corrosion of aluminum, stainless steel, and copper, which have excellent corrosion resistance. These metals corrode in certain environments. Learn the environments in which corrosion occurs so you can take precautions.
Aluminum is light, strong, and resistant to rust, and is used in a variety of fields, including household goods, construction materials for high-rise buildings, ships, vehicles, aircraft, and electrical equipment (Fig. 1). However, high-purity pure aluminum (purity of 99.00% or higher) has low strength and is used for members and parts that do not require mechanical strength. To increase strength, alloying elements are added.
Figure 1: Examples of aluminum productsA highly protective oxide film (passive film) with self-healing ability is formed on the surface of aluminum. This makes aluminum highly resistant to corrosion. The oxide film consists of aluminum oxide Al2O3 and aluminum oxide hydrate Al2O3・It consists of xH2O and is stable in the pH range of 4 to 8.5.
The form of aluminum corrosion is roughly divided into uniform corrosion and localized corrosion. Uniform corrosion occurs when the oxide film dissolves uniformly, and localized corrosion such as pitting occurs when the oxide film is locally destroyed. Uniform corrosion of aluminum occurs in acid or alkaline solutions. Metals that corrode in both acidic and alkaline solutions are called amphoteric metals. Corrosion rate increases with decreasing (below 4) or increasing (above 9) pH (Fig. 2).
Figure 2: Relationship between aluminum corrosion rate and pHOur living environment is maintained at pH 7, near neutrality. Under this environment, the aluminum oxide film is stable and does not corrode. However, if chloride ions are present, ...
>>Read the continuation of Part 2 Chapter 1 (PDF download)
Stainless steel is a corrosion-resistant material that is used in a variety of situations, including household goods, various devices, and plant materials (Fig. 3). Refers to a ferrous alloy containing 11-12% or more of chromium, which does not corrode at all in an atmospheric environment. Typical stainless steels are ferritic or martensitic, which consist of iron and chromium, and austenitic, which consists of iron, chromium and nickel.
Figure 3: Examples of stainless steel productsThe corrosion resistance of stainless steel is demonstrated by the protective oxide film. The oxide film is formed by chromium oxide and chromium hydroxide, which have high self-repairing ability, and is stable in a wider pH range than the aluminum oxide film.
Like aluminum, corrosion of stainless steel is roughly divided into uniform corrosion and localized corrosion. Uniform corrosion occurs in an acid solution environment with extremely low pH. In an environment containing a large amount of chloride ions (halogen ions) such as seawater, localized corrosion such as pitting and crevice corrosion occurs. Recently, pitting corrosion resistant stainless steel (increased chromium concentration in the alloy and addition of molybdenum as an alloying element), which exhibits sufficient corrosion resistance even in seawater, is also used.
A note about stainless steel...
>>Read more of Chapter 2 (PDF download)
Copper is soft and easily conducts electricity and heat, and is used in industrial machinery, transportation machinery, and household goods (Fig. 5). Stable corrosion products (copper oxide, copper hydroxide, basic copper carbonate, etc.) are formed on the surface of copper and copper alloys. Although it is different from passive film, it is insoluble (insoluble in water) and corrosion resistant rust.
Figure 5: Examples of copper productsCopper and copper alloys exhibit a more noble potential than hydrogen in the electrochemical series. Therefore, corrosion reactions based on hydrogen generation do not occur. Also, in water in which oxygen is not dissolved or in a non-oxidizing acid solution, ……
>>Continue reading Part 2 Chapter 3 (PDF download)
Part 3: Environmental Corrosion and Corrosion-Resistant Materials
In the previous article, I explained the corrosion of aluminum, stainless steel, and copper. This time, I will explain about corrosion resistance. Metallic materials are used in a variety of environments, such as freshwater and seawater. Learn about corrosion phenomena and corrosion-resistant materials that occur in each environment.
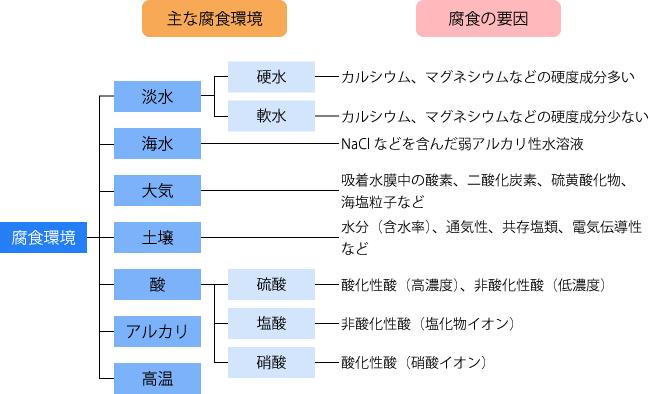
Corrosive environments include freshwater, seawater, atmospheric air, soil, acids, alkalis, and high temperatures. Fig. 1 summarizes the main corrosion environments and corrosion factors. Let's take a closer look at each environment.
Figure 1: Main corrosive environments and corrosive factorsFresh water can be divided into hard water and soft water. Hard water contains a lot of hardness components such as calcium and magnesium, and soft water contains less. In hard water, hardness components precipitate on the steel surface and form an anti-corrosion film. This makes it less corrosive. In soft water, no anti-corrosion film is formed and corrosiveness is high.
Because a stable rust layer (oxide film) is formed on carbon steel in fresh water, it is less corrosive, with a corrosion rate of 0.2 to 0.3 mm/year. The corrosion rate changes depending on the supply rate of oxygen dissolved in freshwater (dissolved oxygen). As the speed of water flow increases, the amount of dissolved oxygen that touches the steel surface also increases, increasing the rate of corrosion.
Tap water, which is a typical fresh water, has...
>>Read the continuation of Part 3 Chapter 2 (PDF download)
Sea water is a weakly alkaline aqueous solution with a pH of 8 containing about 3.5% of salts such as sodium chloride NaCl. The corrosion rate in seawater depends on the dissolved oxygen supply rate, and the average corrosion rate is comparable to that in freshwater. However, localized corrosion such as rusty hump corrosion occurs due to the influence of salinity. Rust-nodular corrosion is corrosion in which rust builds up and forms bumps. Therefore, the use of carbon steel requires painting and cathodic protection. Steel companies have developed and started supplying seawater-resistant steels to which several percentages of alloying elements such as nickel (Ni), chromium (Cr), and copper (Cu) are added.
For seawater corrosion, the necessary countermeasures vary depending on the location and location, such as the mid-sea, tidal zone, splash zone, and oceanic atmosphere. The most corrosive...
>>Part 3 Continue reading Chapter 3 (PDF download)
Atmospheric metal surfaces have a thin film of adsorbed water molecules, even if they appear dry. Oxygen and carbon dioxide in the atmosphere, air pollutants such as sulfur oxides SOx and nitrogen oxides NOx are dissolved in the water film. Therefore, the corrosiveness of metals in the atmospheric environment changes depending on meteorological factors such as humidity, temperature, and rainfall, as well as the type and amount of corrosion-promoting substances such as air pollutants and sea salt particles.
Meteorological conditions determine the corrosiveness of metals in clean air with few corrosion promoters. ……
>>Continue reading Part 3 Chapter 4 (PDF download)
The corrosiveness of metals in the soil environment is determined by the water content (moisture content) and air permeability of the soil, the types and concentrations of coexisting salts, electrical conductivity, and pH. Since microorganisms such as sulfate-reducing bacteria live in the soil, microbial corrosion is also considered. Also consider microbial corrosion. In addition, it is necessary to consider corrosion due to stray currents (leakage currents from railway rails) for buried piping along railway tracks.
An organic insulating coating is used to prevent corrosion of buried pipes. again,……
>>Continue reading Part 3 Chapter 5 (PDF download)
Strong acids such as sulfuric acid, hydrochloric acid, and nitric acid used in the chemical industry are known to be the most corrosive of all environments. This requires the use of high-grade corrosion-resistant metals and alloys such as stainless steel, high-nickel alloys, titanium, zirconium, and tantalum. Alkali is less corrosive than acid and easy to handle, but depending on conditions such as concentration and temperature, it can cause severe corrosion.
The corrosiveness of sulfuric acid varies characteristically with concentration. Concentrated sulfuric acid with a concentration of 60% or more is oxidizing, so carbon steel is passivated and has corrosion resistance. On the other hand, sulfuric acid in low concentrations acts simply as a strong acid and is highly corrosive. On the other hand, lead, which has been used as a sulfuric acid-resistant material, reacts with low-concentration sulfuric acid to produce lead sulfate, which is a corrosion product. This forms a protective film that protects the underlying metal and exhibits corrosion resistance. However, in the case of high-concentration sulfuric acid, lead sulfate is oxidized to lead oxide. Lead oxide easily dissolves in concentrated sulfuric acid and loses its corrosion resistance. Figure 3 shows how the corrosion rate of carbon steel and lead varies with sulfuric acid concentration.
Figure 3: Relationship between corrosion rate of carbon steel and lead and sulfuric acid concentrationSince stainless steel does not passivate in low-concentration sulfuric acid without an oxidizing agent (such as oxygen), it has no corrosion resistance. On the other hand, high-concentration sulfuric acid is oxidizing and passivates, exhibiting good corrosion resistance (Fig. 4). In particular, alloy20 (20Cr-30Ni-3Mo-4Cu), a stainless steel alloy containing a large amount of nickel, molybdenum, and copper, exhibits excellent corrosion resistance to sulfuric acid and is widely used.
Figure 4: Relationship between stainless steel and sulfuric acid concentration……
>>Continue reading Part 3 Chapter 6 (PDF download)
Amphoteric metals (aluminum, zinc, tin, lead, etc.) placed in alkali will corrode severely even at low temperatures. This is because it becomes a metallic acid and dissolves in the same way that iron corrodes in a concentrated alkali. Alkalis do not contain components responsible for corrosion reduction reactions like the hydrogen ions of acids. Therefore, it is not highly corrosive to carbon steel. At medium temperatures and in medium concentrations of alkalis, carbon steel provides adequate corrosion resistance. However, at high concentrations and high temperatures...
>>Continue reading Part 3 Chapter 7 (PDF download)
At high temperatures above 400 to 500°C, metals undergo high-temperature corrosion such as oxidation, sulfidation, carburization, nitridation, and hydrogen damage, depending on the environmental conditions. Hot corrosion is called dry corrosion, whereas corrosion in aqueous solution is called wet corrosion. In high temperature environments...
>>Continue reading Part 3 Chapter 8 (PDF download)
……
>>Continue reading Part 3 Chapter 9 (PDF download)
Part 4: Corrosion Prevention of Steel Materials
In the previous article, we explained corrosion phenomena of metals placed in various environments and corrosion-resistant materials. Corrosion of metals progresses naturally in the natural environment. Therefore, it is necessary to take measures to stop the corrosion of metals or slow down the rate of corrosion. This is corrosion protection. This time, we will explain the anti-corrosion methods for steel materials, such as coating anti-corrosion, cathodic anti-corrosion, and anti-corrosion using anti-corrosion agents.
Covered corrosion protection is a corrosion protection method that covers metals with organic or inorganic films to isolate them from corrosive environments. Types of coating include coating with organic substances such as paint, metal plating, and thermal spraying of metals or inorganic substances (a surface treatment method in which a coating material made into fine particles by heating is sprayed). This time, I will explain painting and plating (zinc plating).
Car bodies made of steel are painted for decoration and corrosion protection. If the steel plate is not painted, it will rust in less than a year. Areas where rainwater easily collects may develop holes in a few years. Painting to protect steel from rust and pitting corrosion is a very effective anti-corrosion method. The following describes paints that are commonly used in various machines, equipment, and plant materials.
The film formed by painting is called a paint film. Substances such as oils and synthetic resins form the coating film. Paints that use oil are called oil-based paints, and paints that use synthetic resin are called synthetic resin paints. There are many types of synthetic resins, including phthalic acid, phenol, chlorinated rubber, vinyl, epoxy, polyurethane, acrylic, and fluorine.
The application of multiple coatings (primer, intermediate and top coats) is a common method of increasing corrosion protection. The purpose of undercoat paint is to adhere to the underlying metal and to prevent rust. Anti-rust paint, zinc-rich paint, epoxy resin, etc. are used. Intermediate paints are used when the undercoat and topcoat do not adhere well. The topcoat has the ability to block the environment, chemical stability against the usage environment, and weather resistance (resistance to deterioration and deterioration due to sunlight). Phthalic acid-based, polyurethane-based, and fluorine-based resin paints are used.
Since each paint has advantages and disadvantages, it is necessary to select an appropriate combination while considering the underlying metal, usage environment, and economic efficiency. Table 1 describes the characteristics of typical paints.
Table 1: Representative paints and characteristics| Paints | Characteristics |
| Zinc rich paint | ・Contains metallic zinc powder ・Even if the paint film is scratched, the sacrificial anodic action of zinc suppresses corrosion of the underlying metal. |
| Epoxy resin | ・Excellent water resistance, chemical resistance, adhesion, and mechanical strength ・Due to poor weather resistance, an appropriate topcoat is required when used outdoors. |
| Phthalic acid resin Polyurethane resin Fluorine resin | ・Shows weather resistance and stable properties over a long period of time |
……
>>Continue reading Chapter 4 Chapter 1 (PDF download)
Cathodic protection is a method of preventing corrosion by manipulating the potential of metals in water or soil by passing an electric current through them. Corrosion of metals in water or soil is caused by current flowing from the metal into the environment. The flowing current is called corrosion current. Corrosion can be prevented by direct current flowing from the environment into the metal against the corrosion current (anti-corrosion current), which stops the outflow of the corrosion current. There are two types of cathodic protection: the sacrificial anode method, in which base metals are electrically contacted, and the external power supply method, in which an external DC power supply is used.
The principle of the sacrificial anode method is...
>>Continue reading Part 4: Chapter 2 (PDF download)
Anti-corrosion agents suppress corrosion by forming anti-corrosion films on metal surfaces in addition to the environment (usually aqueous solutions). The merits are that it is effective in reducing corrosion with a small amount added, is economical at a low price, and is environmentally friendly. Corrosion inhibitors are classified into three types: oxidation-type corrosion inhibitors, precipitation-type corrosion inhibitors, and adsorption-type corrosion inhibitors (Table 2).
Table 2: Types and characteristics of anticorrosive agentsAnticorrosive agents are used in a wide range of applications, including circulating cooling water systems, acid cleaning fluids, and crude oil and natural gas pipelines. The most commonly used...
>>Continue reading Part 4 Chapter 3 (PDF download)
The 5th: What is anti-corrosion design? What is anti-corrosion management?
In the previous article, we introduced anti-corrosion methods such as coating anti-corrosion, cathodic anti-corrosion, and anti-corrosion agents. This time, we will explain anti-corrosion design and anti-corrosion management. Even structures made of superior materials that are compatible with environmental conditions are subject to corrosion damage due to design flaws. In addition, even structures that are constructed with appropriate materials and structures and are used in good condition will gradually corrode from weak points as the period of use increases. Proper design as well as ongoing repairs and maintenance are essential to prevent corrosion.
The know-how necessary to prevent corrosion has been accumulated in the design technology of devices and equipment used in harsh environments such as chemical plants. Anti-corrosion design is a design that takes into account the characteristics of metals and the environment in which they are used to prevent corrosion. Below are 9 key points for anti-corrosion design.
At the design stage, it is necessary to reduce the structural crevices that cause corrosion and prevent crevice corrosion. It is effective to avoid overlapping connections (bolts, screws, etc.) and weld them into an integrated structure (Fig. 1). Materials with high corrosion resistance are applied to structurally unavoidable crevices such as connecting pipes and joints. It is also effective to change the gasket material of the connection part from a porous material to a highly water-repellent tetrafluoride resin.
Figure 1: From lapped joints (bolted) to welded jointsBy reducing residual stress and operating stress, stress corrosion cracking and corrosion fatigue cracking can be suppressed. Residual stress caused by cold working or welding is a major factor in stress corrosion cracking. Heat and anneal to remove residual stress.
Since the critical stress at which corrosion fatigue cracking no longer occurs is low, measures to prevent cracking by lowering the absolute value of the operating stress are not effective. Therefore, by improving the shape of the product, for example by making it round instead of making sharp notches, the operating stress is dispersed and cracking is prevented (Fig. 2). This method is very effective and important for design.
Fig. 2: Improved shape disperses stress and prevents cracks Abrasion corrosion occurs due to mechanical action such as abrasion corrosion. To avoid these, it is essential to take countermeasures at the design and construction stages. Take measures such as lowering the flow velocity, making the bends in the pipe gentle, and not creating local unevenness that disturbs the flow.……
>> Read the continuation of Part 5 Chapter 1 (PDF download)
Daily anti-corrosion management is essential for structures and equipment to perform as designed without causing malfunctions or accidents. The basis of anti-corrosion management is careful observation and acquisition of quantitative information. If you can observe the remaining wall thickness and notice any changes, you can suspect corrosion. Appropriate observation and maintenance will enable repair before corrosion progresses. Two points of anti-corrosion management are explained below.
By carefully observing the surface of facilities and equipment from the outside, signs of corrosion and changes can be noticed. If the surface is painted, when corrosion begins, rust juice flows out, so you can tell at a glance that corrosion has begun (Fig. 5).
Fig. 5: Rust juice flowing out due to corrosionThe parts that are difficult to see from the outside are disassembled and observed by looking into them. Areas to be observed intensively are areas that are difficult to process or paint, such as corners of structures and bolted areas. If any corrosion is found, repair paint will be applied as soon as possible.
……
>> Read the continuation of Part 5 Chapter 2 (PDF download)
6th: Corrosion Analysis and Corrosion Case Database
In the previous article, I explained anti-corrosion design and anti-corrosion management. This is the final time. We will explain the cause analysis of corrosion and the database of corrosion cases. When a corrosion accident occurs, it is necessary to quickly analyze the cause and take countermeasures. Using a database at that time allows us to refer to similar corrosion cases that have occurred in the past, which is useful for verifying causes and planning countermeasures.
The purpose of corrosion analysis is to clarify the cause of corrosion and take the most appropriate countermeasures while considering economic efficiency. Corrosion in equipment is typically analyzed in five steps.
Before the analysis, check the function and role of the part where corrosion occurs in the equipment.
Check the material composition and metallographic structure where corrosion occurred. Also check the environment in which the material was used. The key point is to check the environment both when the equipment is in operation and when it is out of service.
……
>>Continue reading Chapter 6 Chapter 1 (PDF download)
Many cases of material damage are caused by corrosion. There is also a document that about 3/4 of damage cases are caused by corrosion (reference: Society of Chemical Engineers SCE・Net Device Materials Study Group, Plant Material Damage Cases, 2017). In particular, many cases of corrosion of stainless steel have been reported. This is because stainless steel, which has excellent corrosion resistance, is easily used in corrosive environments. Here are two cases of stress corrosion cracking in stainless steel.
Hot water at 90°C flows inside the heating pipe of the dryer, and the moisture content of synthetic resin products flowing outside the heating pipe is dried from 20% to 1-2%. Fig. 1 shows stress corrosion cracking in the stainless steel (SUS316L) heating tube of the dryer.
Figure 1: Stress corrosion cracking in a stainless steel (SUS316L) heating tube (left: heating tube surface, right: heating tube cross section) (Source: Society of Chemical Engineers, SCE・Net Device Materials Study Group, Plant Material Damage Case Study, 2017) Year, case number TKW-059)Let's take a closer look at this corrosion cracking case. Corrosion cracking occurred when the heating tube was used for about two months. The morphology of the crack is a branched transgranular crack. In the residual stress measurement results, a tensile stress value of about 20 kg/mm2 in the circumferential direction and about 10 kg/mm2 in the axial direction was measured. The heating tube is surface-polished to prevent foreign matter from adhering to it. In a reproduction test, we were able to confirm the occurrence of residual stress due to this surface polishing. Possible countermeasures include improving the surface polishing method and removing residual stress by heat treatment.
……
>> Read the continuation of Part 6 Chapter 2 (PDF download)
The database of damage cases is a database of damage cases caused by corrosion that occurred in the past. Today, many companies are building their own systems, and it is now possible to share information within departments or across the company via intranets. If the following items are covered, it can be said that it is a useful database.
On the other hand, it takes time to accumulate useful examples. In addition, manpower is required for database construction and maintenance. If it is difficult to create the database in-house, consider purchasing the database from outside the company. We have collected damage cases in each field and created a database of case examples for sale both in Japan and overseas. As an example of a database with many cases of damage due to corrosion,……
>>Continue reading Chapter 6 Chapter 3 (PDF download)
